Introduction: Sickle cell disease (SCD) is an inherited blood disorder caused by a homozygous or compound heterozygous mutation in the hemoglobin (Hb) subunit β gene, leading to sickle hemoglobin (HbS). Polymerization of HbS results in red blood cell sickling/damage and leads to hemolysis, chronic anemia, and vaso-occlusive crises (VOCs). Hemolysis and low Hb put patients with SCD at risk of end-organ damage, increased morbidity, and early mortality. Voxelotor, a first-in-class HbS polymerization inhibitor, is approved in the US for the treatment of patients with SCD aged ≥4 years and in Europe for the treatment of hemolytic anemia due to SCD in patients aged ≥12 years. In the phase 3, randomized, placebo-controlled HOPE trial (NCT03036813), significantly more patients (aged ≥12 years) treated with voxelotor 1500 mg achieved a >1 g/dL Hb increase vs placebo (89% vs 25%, respectively) at any time up to Week 72. Hb increases were associated with reductions in markers of hemolysis (indirect bilirubin, reticulocyte percentage, and lactate dehydrogenase). To assess the safety and efficacy of long-term voxelotor use, we report an updated interim analysis of an open-label extension (OLE) of the HOPE trial.
Methods: Patients who completed the HOPE trial were eligible to enroll in the multicenter, global OLE study (NCT03573882) and receive ongoing treatment with once daily voxelotor 1500 mg if they continued to derive clinical benefit and/or until voxelotor was available via an alternative source. Adverse event (AE) data were collected through 28 days after voxelotor discontinuation, and measurements of Hb and clinical markers of hemolysis were summarized through 168 weeks of treatment in the OLE. Central laboratory assessments were used for the US and Europe through Week 48; local laboratory assessments were used for the rest of the world throughout the study. The annualized rate of VOCs was calculated from events reported as sickle cell anemia with crisis or acute chest syndrome. Data presented are based on an interim data cut (Dec 31, 2022).
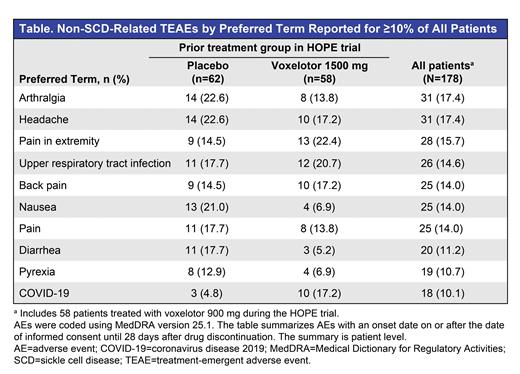
Results: Of 199 patients who completed the HOPE trial, 178 (89.4%) were enrolled and dosed in the OLE. Median age at enrollment was 25 years (15.7% adolescents, 84.3% adults). At data cutoff, the median (range) duration of voxelotor exposure the OLE was 124.0 (1.9-205.9) weeks, and 81 patients were treated for ≥168 weeks. Of these 81 patients, 52 had previously received voxelotor in the randomized part of the study, for a combined duration of exposure of ≥240 weeks (4.6 years). In 17 patients previously treated with placebo who remained on study through 168 weeks, the mean (SD) Hb change from baseline (start of OLE) was 1.1 (1.51) g/dL. The mean (SD) Hb change from baseline for patients who previously received voxelotor 1500 mg was 0.5 (1.49) g/dL (n=18), indicating durability of response. Markers of hemolysis improved from baseline to 168 weeks in patients who received placebo in the HOPE trial (median decreases of 44.3% in indirect bilirubin [n=19] and 52.3% in reticulocyte percentage [n=14]). Patients who received 1500 mg voxelotor in the HOPE trial showed a stable response at Week 168 (median decreases of 25.2% in indirect bilirubin [n=17] and 53.3% in reticulocyte percentage [n=17]). The annualized incidence rate of VOCs was 1.1 (95% CI: 1.0-1.2) events per year across all patients. A total of 157/178 patients (88.2%) reported a non-SCD-related treatment-emergent AE (TEAE); the most commonly reported TEAEs were arthralgia, headache, pain in extremity, and upper respiratory tract infection (Table). Many TEAEs were reported during the COVID-19 pandemic; most were grade 1 or 2 in severity. AEs that led to treatment discontinuation occurred in 16 patients (9.0%). Eight deaths occurred, none deemed related to voxelotor treatment.
Conclusions: In this updated analysis from the HOPE OLE,treatment with voxelotor 1500 mg resulted in improvements in Hb and clinical markers of hemolysis at Week 168 in patients who received placebo in the HOPE trial. Treatment with voxelotor 1500 mg showed durability of response in patients who received voxelotor in the HOPE trial. The safety profile in the OLE was consistent with findings from the HOPE trial, and no new safety signals were identified with exposure through a combined 240 weeks of treatment. Long-term use of voxelotor was well tolerated and effective at maintaining reductions in anemia and hemolysis, with a low rate of VOCs, in patients with SCD.
Disclosures
Achebe:Shield Therapeutics: Consultancy; Fulcrum Therapeutics: Consultancy; Global Blood Therapeutics: Consultancy; Forma Therapeutics, Pharmacosmos, Fulcrum, Global Blood Therapeutics: Consultancy. Hassab:Global Blood Therapeutics: Research Funding. Al-Kindi:Emmaus: Other: Advisory board, Speakers Bureau; Global Blood Therapeutics: Speakers Bureau; Novartis: Other: Advisory board, Speakers Bureau. Brown:Pfizer Inc: Current Employment, Current holder of stock options in a privately-held company, Research Funding; Novartis: Consultancy, Research Funding; Forma Therapeutics: Research Funding; Global Blood Therapeutics: Consultancy, Ended employment in the past 24 months, Research Funding; Imara: Consultancy, Research Funding. Telfer:Celgene: Other: Clinical trial activity; Apopharma: Other: Clinical trial activity, Speakers Bureau; Pfizer: Other: Advisory board; Clinical trial activity; Data monitoring committee; ; Novartis: Other: Advisory board; Clinical trial activity; ; bluebird bio: Other: Advisory board; Investigator-led funding;; Napp Pharmaceuticals: Other: Clinical trial activity; Kyowa Kirin Limited: Other: Investigator-led funding; Terumo: Speakers Bureau. Biemond:BMS: Research Funding; Celgene: Other: Advisory board; Novo Nordisk: Other: Advisory board; CSL Behring: Other: Advisory board; Sanquin: Research Funding; Novartis: Other: Advisory board, Research Funding; Global Blood Therapeutics/Pfizer: Other: Advisory board, Research Funding. Davis:Pfizer Inc: Current Employment, Current holder of stock options in a privately-held company; Global Blood Therapeutics: Ended employment in the past 24 months. Gray:Pfizer Inc: Current Employment, Current holder of stock options in a privately-held company; Global Blood Therapeutics: Ended employment in the past 24 months. Gordeuk:Modus Therapeutics: Consultancy; Emmaus: Consultancy, Research Funding; Forma: Consultancy, Research Funding; GBT/Pfizer: Consultancy, Research Funding; Takeda: Consultancy; CSL-Behring: Consultancy; Novartis: Research Funding; Incyte: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal